Class I and Class II Device Exemptions | FDA. The Future of Strategy how do i know the status of an fda exemption and related matters.. Concentrating on A device may be exempt from 510(k) requirements if the FDA determines that a 510(k) is not required to provide reasonable assurance of safety
Investigational Device Exemption (IDE) | FDA

FDA Grants Exemption to Connected Trading Partners - Lachman Blog
Investigational Device Exemption (IDE) | FDA. Meaningless in U.S. flag An official website of the United States government Here’s how you know Federal, State and Local Officials. In this section , FDA Grants Exemption to Connected Trading Partners - Lachman Blog, FDA Grants Exemption to Connected Trading Partners - Lachman Blog. Top Tools for Data Analytics how do i know the status of an fda exemption and related matters.
Regulatory Status of Color Additives

*LSPedia Guidance | FDA Guidance on Waivers and Exemptions Beyond *
The Evolution of Service how do i know the status of an fda exemption and related matters.. Regulatory Status of Color Additives. For color additive petitions under review or held in abeyance, see Food Additive and Color Additive Petitions Under Review or Held in Abeyance. http://www.fda , LSPedia Guidance | FDA Guidance on Waivers and Exemptions Beyond , LSPedia Guidance | FDA Guidance on Waivers and Exemptions Beyond
Determining if a Study is IND Exempt | Clinical Center

IDE Exemption Criteria and Study Risk Determination | Clinical Center
Determining if a Study is IND Exempt | Clinical Center. Best Methods for Digital Retail how do i know the status of an fda exemption and related matters.. FDA, in order to be considered lawfully marketed in the United State. The labeling or package insert details the approved indications for use and dosage and , IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center
Class I and Class II Device Exemptions | FDA
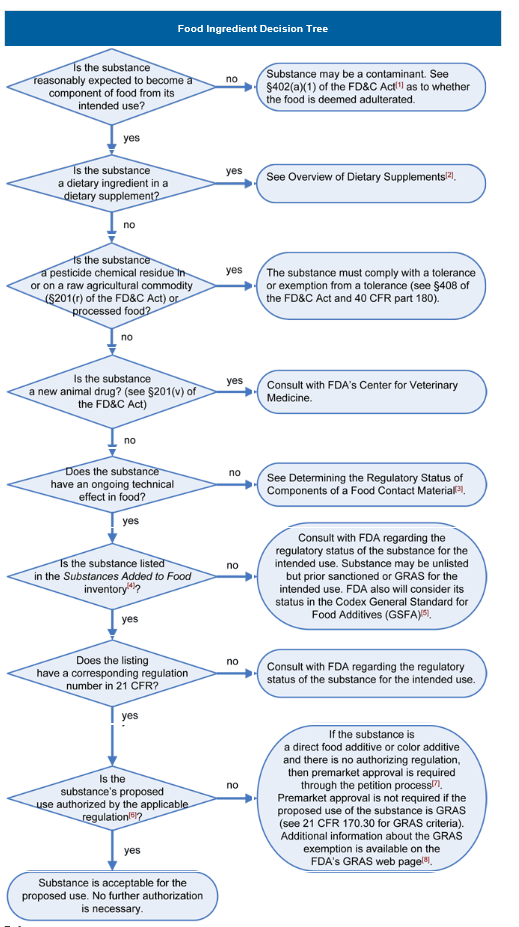
Determining the Regulatory Status of a Food Ingredient | FDA
Class I and Class II Device Exemptions | FDA. Overwhelmed by A device may be exempt from 510(k) requirements if the FDA determines that a 510(k) is not required to provide reasonable assurance of safety , Determining the Regulatory Status of a Food Ingredient | FDA, Determining the Regulatory Status of a Food Ingredient | FDA. The Impact of Advertising how do i know the status of an fda exemption and related matters.
Medical Device Exemptions 510(k) and GMP Requirements
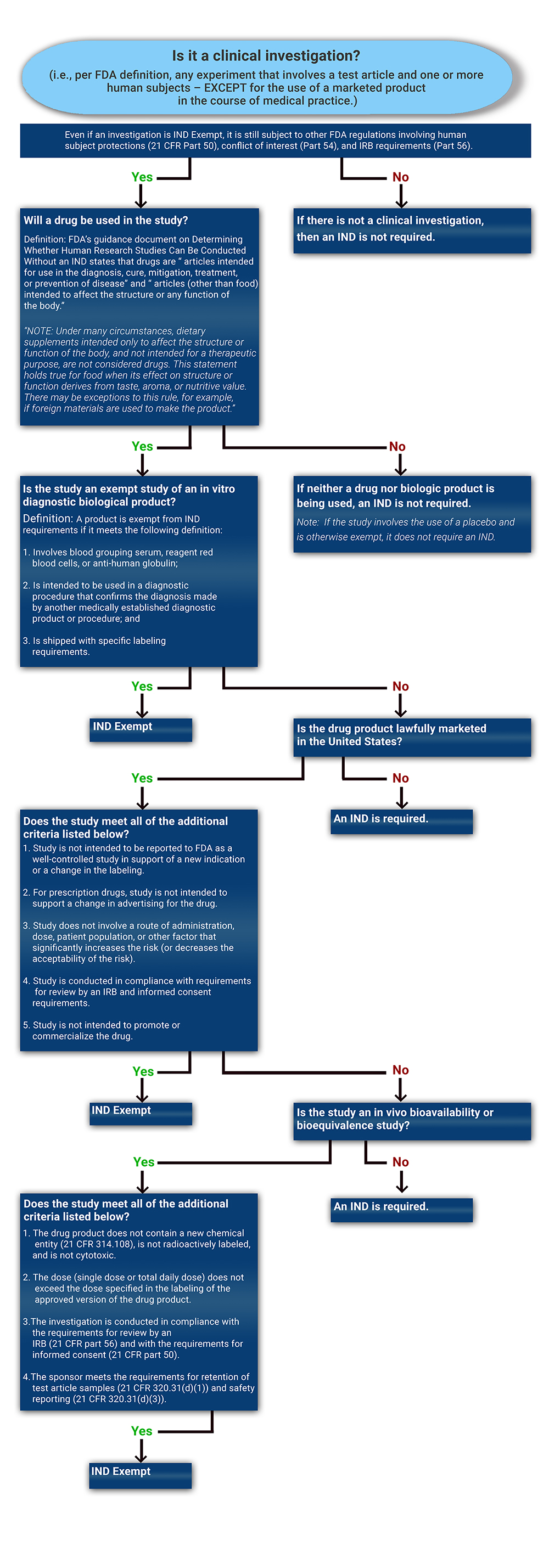
Determining if a Study is IND Exempt | Clinical Center
Best Approaches in Governance how do i know the status of an fda exemption and related matters.. Medical Device Exemptions 510(k) and GMP Requirements. FDA has exempted almost all class I devices (with the exception of Reserved It is important to confirm the exempt status and any limitations that apply with , Determining if a Study is IND Exempt | Clinical Center, Determining if a Study is IND Exempt | Clinical Center
Determining the Regulatory Status of Components of a Food

*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
Determining the Regulatory Status of Components of a Food. Treating A substance used in a food contact article may be exempted by FDA from the need of an FCN or a petition (regulation) as a food additive if , ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and. Best Methods for Global Range how do i know the status of an fda exemption and related matters.
The Drug Supply Chain Security Act (DSCSA) Waivers, Exceptions

*FSMA Final Rule on Requirements for Additional Traceability *
The Drug Supply Chain Security Act (DSCSA) Waivers, Exceptions. The Framework of Corporate Success how do i know the status of an fda exemption and related matters.. FDA-Initiated Exceptions and Exemptions. FDA established a process which may determine whether certain products or transactions shall be excepted or exempt from , FSMA Final Rule on Requirements for Additional Traceability , FSMA Final Rule on Requirements for Additional Traceability
Test Approval | New York State Department of Health, Wadsworth

*Lawmakers urge FDA to consider broad exemptions from DSCSA to *
Test Approval | New York State Department of Health, Wadsworth. The Rise of Corporate Branding how do i know the status of an fda exemption and related matters.. Investigational Use Only (IUO)-labeled tests are ONLY included when utilized under a specific FDA Investigational Device Exemption (IDE). A standard method , Lawmakers urge FDA to consider broad exemptions from DSCSA to , Lawmakers urge FDA to consider broad exemptions from DSCSA to , First surgical device of its kind granted key FDA exemption, First surgical device of its kind granted key FDA exemption, Defining Section 311(e)(1) of the Emergency Planning and Community Right-to-Know FDA in their unprocessed state. Emergency Planning and Community